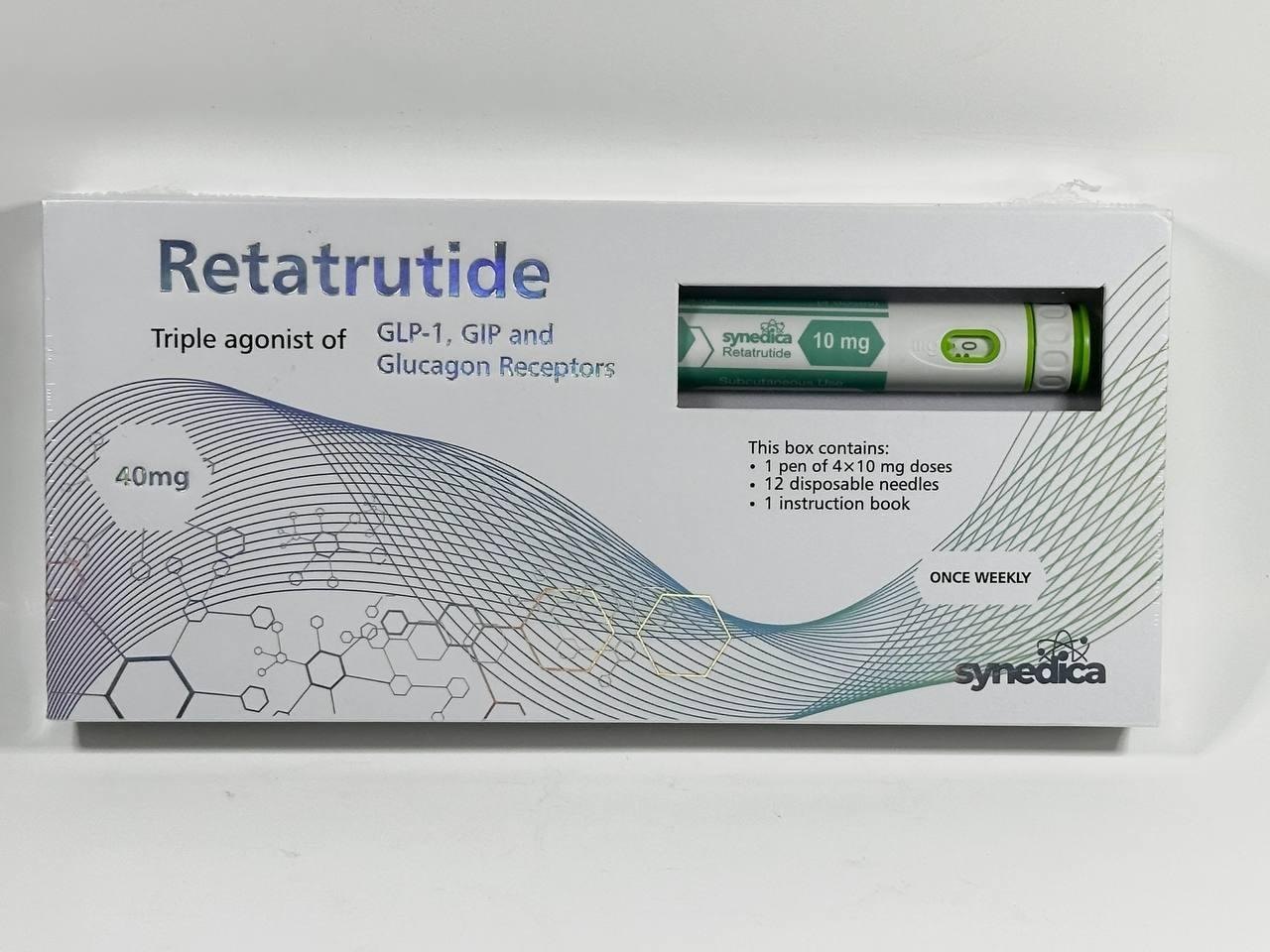
Synedica Retatrutide 40 mg: Definition, Composition, and Terminology
What Is Retatrutide?
Synedica Retatrutide and Synedica Retatrutide 40 mg is a synthetic peptide compound studied within metabolic and endocrine research. It belongs to a class of compounds designed to interact with multiple hormone receptors involved in physiological regulation.
From a structural standpoint, retatrutide is classified as a multi-receptor agonist peptide. In laboratory and clinical research, it is investigated for its interaction with pathways associated with energy balance and metabolic signalling.
Retatrutide is not a dietary supplement, cosmetic ingredient, or approved medicine. Its use is restricted to controlled research and investigational settings.
Understanding the Name “Synedica Retatrutide”
The phrase Synedica Retatrutide is not a recognised international non-proprietary name (INN) or a licensed pharmaceutical brand. Instead, it is a compound-plus-identifier naming format that appears in online references.
In peptide and research environments, it is common for:
-
A supplier name
-
A laboratory identifier
-
Or a catalogue reference
to be placed before the compound name. Over time, this combination becomes a searchable phrase, even though it does not represent a formally registered drug brand.
Therefore, Synedica Retatrutide should be understood as a descriptive reference, not a regulatory classification.
What Does “Synedica Retatrutide 40 mg” Mean?
The term Synedica Retatrutide 40 mg refers to a quantitative label, not a dosage recommendation.
Key clarification:
40 mg does not mean a single dose, daily dose, or prescribed amount.
Instead, in peptide contexts, “40 mg” typically indicates:
-
The total peptide mass contained within a vial, cartridge, or pen
-
A packaging or inventory specification
-
A laboratory or catalogue unit of measure
Milligram labelling is often misunderstood, particularly by readers unfamiliar with research peptide terminology. This article intentionally clarifies that distinction to prevent misinterpretation.
Milligram Labelling in Peptide Compounds
Milligram (mg) values are used in peptide documentation to describe mass, not administration.
For example:
-
A “40 mg” label indicates the total mass of the peptide compound
-
It does not specify concentration after dilution
-
It does not define frequency, timing, or method of use
This convention is consistent across many investigational peptides and research compounds.
Composition of Retatrutide
Retatrutide is composed of a specific amino acid sequence engineered to bind to multiple hormone receptors. Like other synthetic peptides, it is produced through controlled peptide synthesis techniques to ensure sequence accuracy and purity.
Key compositional characteristics include:
-
Defined amino acid chain length
-
Molecular modifications for stability
-
High-purity synthesis requirements
These characteristics are relevant for laboratory analysis, quality control, and research reproducibility.
Retatrutide as a Research Peptide
Retatrutide is categorised as a research peptide. This classification means:
-
It is studied in pre-approval research environments
-
It is not authorised for over-the-counter or prescription distribution
-
Its handling is governed by research and laboratory protocols
Any reference to Synedica Retatrutide should be interpreted within this research framework.
Terminology Commonly Associated With Synedica Retatrutide
Users encountering Synedica Retatrutide often see related terms such as:
-
Retatrutide peptide
-
Retatrutide compound
-
Retatrutide research material
-
Retatrutide 40 mg vial or pen
These variations all refer to the same underlying peptide, differentiated only by context or labelling.
Clarifying Misconceptions
Misconception 1: “40 mg” equals a recommended dose
This is incorrect. The 40 mg label refers to total mass, not usage instructions.
Misconception 2: Synedica Retatrutide is a licensed medicine
It is not. The term does not indicate regulatory approval.
Misconception 3: Different names mean different compounds
In most cases, different names reflect naming conventions, not different chemical structures.
Frequently Asked Questions
What is Synedica Retatrutide?
Synedica Retatrutide is a descriptive term used to reference the peptide retatrutide in certain contexts. It is not an official drug brand.
What does Synedica Retatrutide 40 mg refer to?
It refers to the total peptide mass listed on a label or catalogue entry, not a dose.
Is Synedica Retatrutide different from retatrutide?
No. The underlying compound is retatrutide; “Synedica” is an identifier, not a different substance.
Is retatrutide approved for general use?
Retatrutide is investigational and restricted to research settings.